Abstract
INTRODUCTION
Lymphoma cells are dependent on the tumor microenvironment (TME) for their survival and proliferation. Dissection of TME composition provides insight into lymphomagenesis, better prognostication, and enhancement of therapeutic options. Flow cytometry provides a robust approach for single-cell analysis. Nonetheless lack of well-defined comparator groups and/or a robust, reproducible approaches for analyzing the multidimensional data often limited such studies. In the current study, we analyzed the T cell background on a large reference set of follicular hyperplasia (FH) lymph node samples utilizing a robust standardized high dimensionality flow cytometry and a novel reproducible analytical pipeline. We then compared this baseline reference set with tumor infiltrating T cell subsets in follicular lymphoma (FL; in treatment naïve FL-NA and relapsed/refractory FL-RR sets).
METHODS
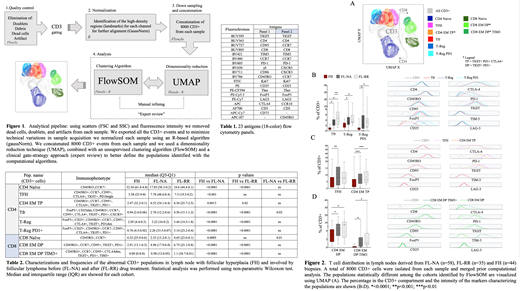
On behalf of the imCORE Network we analyzed 44 FH and 81 FL (35 FL-NA and 44 FL-RR) with 2 standardized flow cytometry panels (23 antigen/18-color) (Table 1). We evaluated the T cell subset distribution as well as immune checkpoint expression (TIGIT, TIM3, PD-1, CD96, LAG3, CTLA4, CD73) within analytically defined T cell clusters. Analysis was performed using semiautomated multi-step analytical pipeline as outlined in Figure 1. Using standardized instrument settings and an R-based algorithms (gaussNorm) to minimize technical variations in sample acquisition we analyzed the T-cells subpopulations using a dimensionality reduction technique (UMAP), combined with an unsupervised clustering algorithm (FlowSOM). Statistical analysis was performed using non-parametric Wilcoxon test.
RESULTS
In the 3 cohorts, several marked differences in the composition T cells subsets were observed. Compared to FH the FL lymph nodes were depleted for CD4 & CD8 naïve subsets (Table 2) and were characterized by an immune suppressive microenvironment enriched in specific subsets of activated T regulatory cells and exhausted memory effector cells. The pool of CD8 naïve cells was restored in FL-RR cases (Table 2).
FL-NA nodes showed enrichment of T follicular regulatory cells (Tfr; Table 2) (0.9% vs 2.7%, p<0.0001) expressing FoxP3, dim to negative CD25, CD45RO, TIGIT, PD1, CTLA and CXCR5 (Table 2) and activated regulatory T cells (T-Reg) characterized by a highly suppressive phenotype CD25+, CTLA4+, TIGIT+ and PD1+ (Fig. 2B). Moreover, Tfr compartment was further expanded in relapsed/refractory FL cases (2.7% vs 4.5%, p=0.02).
In association with the increase of Tfr cells, the concentration of CTLA4+, TIGIT+, PD1bright T follicular helper cells (Tfh) was reduced in FL-RR compared to FL-NA (Fig. 2C).
The Tfr/Treg expansion was also associated with a marked increase in exhausted memory T cells in both CD4 and CD8 compartments (CD4 EM TP and CD8 EM DP, Table 2). In FL isolates the CD4 compartment was characterized by the expression of triple positive (CTLA4, TIGIT, PD1) phenotype in EM cells while the memory CD8 cells overexpressed TIGIT and PD1 (Fig. 2D). A smaller subpopulation of memory CD8 cells, almost undetectable in FH samples, characterized by the expression of CTLA4, PD1, TIGIT and TIM3 is expanded in FL isolates (Fig. 2D).
CONCLUSION
Our data suggest that change in balance between TFH and Tfr may lead to more aggressive therapy resistant disease in FL. The interplay between TFH and Tfr, has been postulated to shape the immune response in FL with TFH promoting germinal center formation and Tfr inhibiting TFH and follicular effector T cells. While both TFH and Tfr compartments are expanded in FL, in the relapsed/ refractory FL cases, the Tfr compartment is further expanded at the expense of TFH leading to more immunosuppressive background. Furthermore our study suggests a rational way of designing immune checkpoint inhibitor studies in FL. Effector memory T-cells in FL isolates show an exhausted phenotype characterized by the expression of the inhibitory receptors CTLA4, TIGIT, PD1 in the CD4 compartment, and TIGIT, PD1 in the CD8. In addition, the noteworthy expansion of TIM3+ memory CD8 cells in FL. Targeting these most highly expressed checkpoints in FL alone or in combination may provide an avenue for rational trial design.
Roshal: Celgene: Other: Provision of services; Auron Therapeutics: Other: Ownership / Equity interests; Provision of services; Physicians' Education Resource: Other: Provision of services. Dogan: Physicians' Education Resource: Honoraria; Peer View: Honoraria; Roche: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy; EUSA Pharma: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal